

DERIVO® peripher
Embolisation Device
Braided self-expanding flow diverter
- Reducing flow velocity within aneurysm while preserving side branches patency
- Precise positioning as well as atraumatic wall apposition due to highest flexibility
- Excellent visibility due to nitinol composite wires with platinum core
- Treatment of tortuous anatomies and distal located aneurysms
- Compatibility with small and flexible delivery systems (0.027‘‘ – 0.039‘‘ ID)
- To be used in combination with NeuroSlider® 27 39 DLC as delivery catheters and NeuroBridge® 52 65 as intermediate catheter
Note: Magnetic Resonance Imaging (MRI) Information
Non-clinical tests have shown that the Acandis implant is suitable for MR-examination. After implantation, patients can be safely scanned with a static magnetic field of 3 Tesla. The MR imaging quality may be affected if the implant is located in the area of interest. Optimisation of the imaging parameters is recommended. For further information on MRI compatibility please consult the instructions for use of the respective product.
Clinical Support
Acandis® offers an individual Sizing Support Service as well as hands-on training
with patient-specific silicone model and selected DERIVO® peripher Embolisation Device
in preparation for the planned intervention.
Contact
Clinical Support (clinical-support@acandis.com)
Clinical Experience
with DERIVO® peripher Embolisation Device
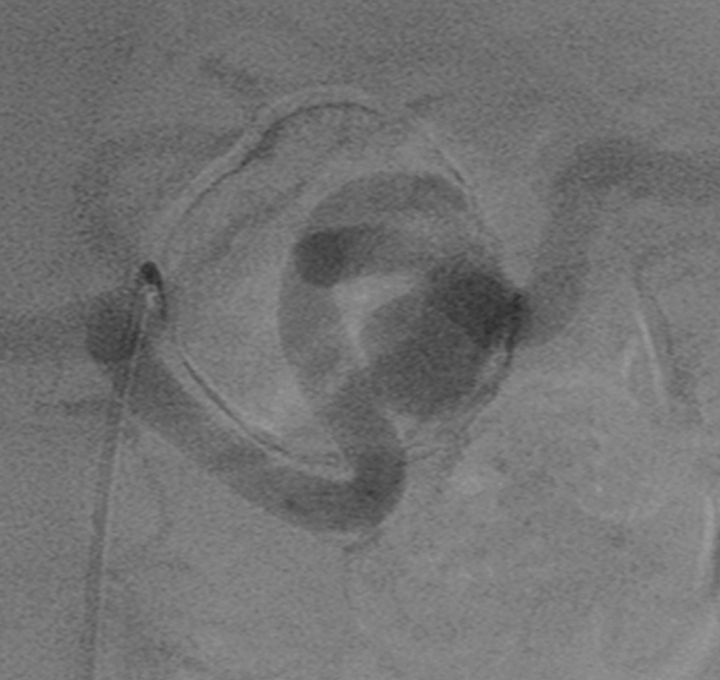
Pre Treatment
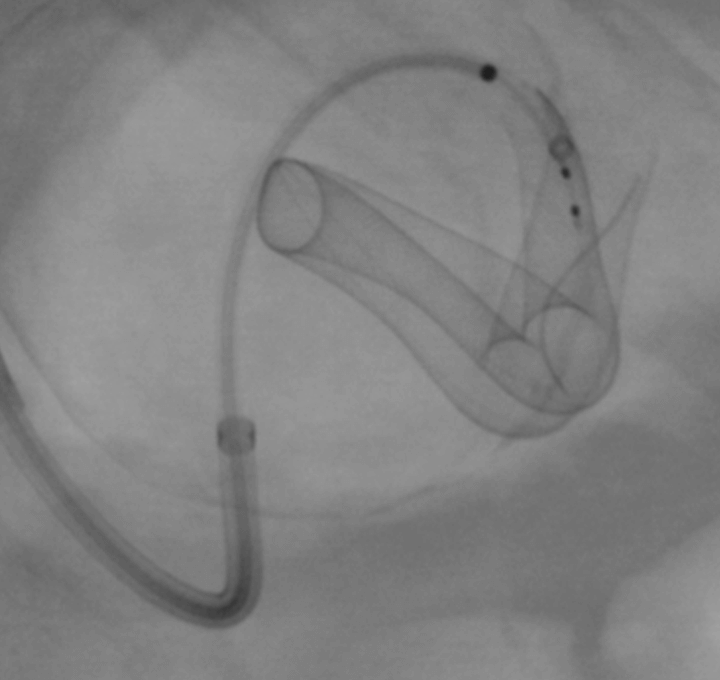
During Deployment
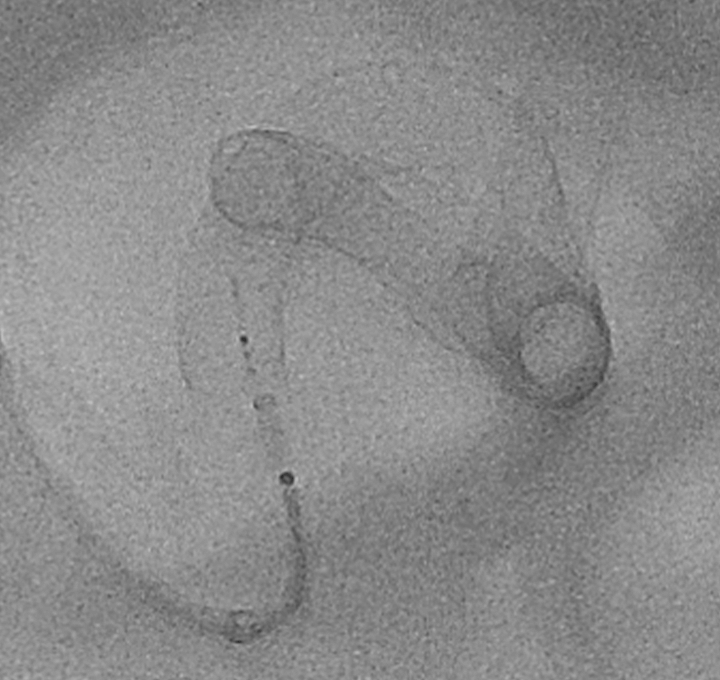
Post Treatment
Images are courtesy by
Prof. Dr. Peter Minko, Universitätsklinikum Düsseldorf, Germany
Publications
2023
Spüntrup, E. et al.Behandlung eines großen Aneurysmas der Arteria hepatica propria mit einem Flowdiverter -
Erste Fallbeschreibung nach der Zulassung für die Behandlung von Viszeralaneurysmen
Gefässchirurgie Volume 28, pages 249–254, (2023)
DOI 10.1007/s00772-022-00958-2
2022
Semeraro, V. et al.Flow-diverter treatment for renal artery aneurysms. One year follow-up of a multicentric
preliminary experience
Diagnostic and Interventional Radiology 2022, 28(6):609-615
DOI 10.5152/dir.2022.211015
Disclaimer:
Please consult the Instructions for Use for all indications, contraindications, warnings, cautions as well as possible adverse effects. Acandis® products are to be used exclusively by trained medical professionals. Orders are taken only in regions where the product is approved.
Please contact an Acandis® representative for product availability.